U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements
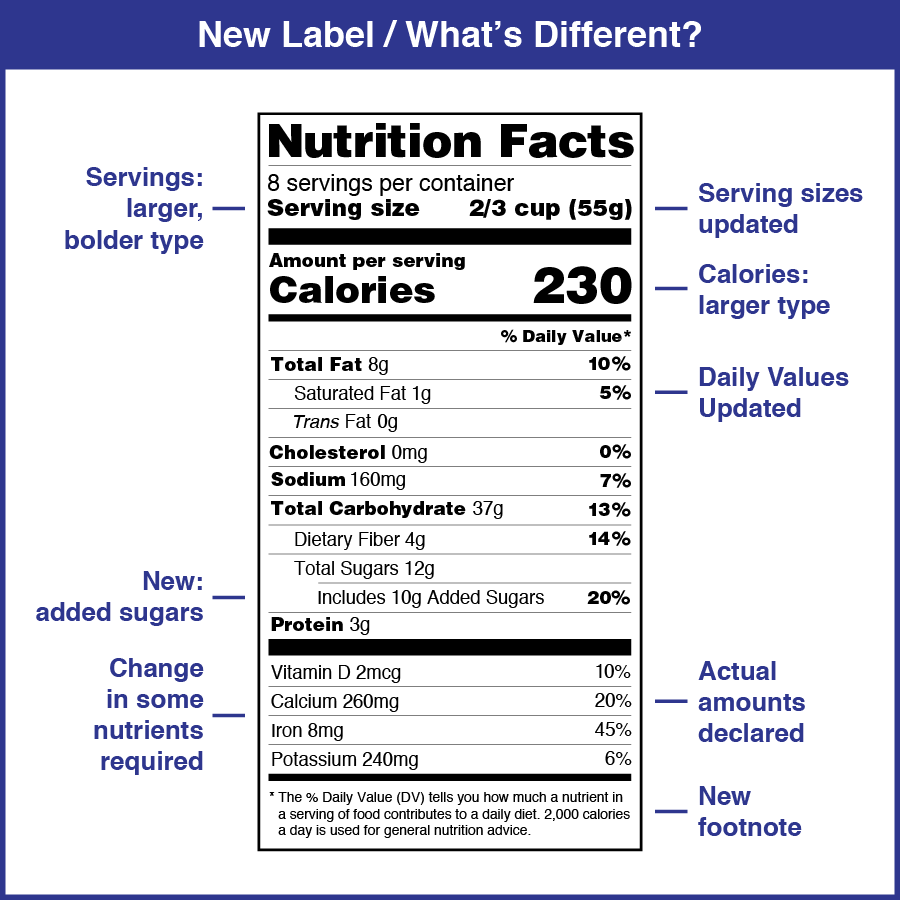
On May 20th , 2016, the Food and Drug Administration published new FDA labeling regulation for foods, beverages and dietary supplements involving Nutrition Fact table, daily values, serving size, etc. According to the new FDA regulation, labels of food, beverage and dietary supplements must contain Nutrition Facts information that comply with new FDA requirements in respect to the labels formatting, nutrient names and the exact amounts, and percent daily value calculation.
Labeling issues are often the primary cause of delays in FDA release andimport detentions. FDA Listing Inc. conducts label and ingredient reviews for foods and dietary supplements in accordance with the FDA labeling guidelines and regulations in the following order.
Nutrition Facts Panel: Calculation and determination of the serving size in the U.S. metricsystem and preparation of nutrition facts panel.
Ingredient Statement: Review and advisory for changes to the order or the nomenclature of the statement in order to follow a FDA compliant and consumer-friendly ingredient statement.
Labeling Claims: Review and guidance for nutrient content claims, organic claims, health claims and other regulated (i.e pasteurized, natural, fresh, etc) claims that appear on the product label.
Label Layout and Format: Redesign and suggestion of a finished label that fits into the product profile in terms of size, shape, layout and formatting.
Other Considerations include: Allergen statement, structure and function claims, nutrient claims and more.
FDA Listing Inc. labeling regulation experts who have an extensive background with providing FDA compliant labeling requirements will first analyze your current label by cross-checking against relevant U.S. federal regulatory databases and FDA labeling guidelines. This will encompass the modification considerations. Next, we will provide you with comprehensive detailed product-specific labeling report.
Service & Fees: